Quoted from: Qiuxian Yang, Gao Germany (Guangzhou Research Institute of Nonferrous metals, Guangzhou 510651)
CLC number: TP923.13 Document code: A Article ID: 1671-9492 (2005) 06-0037-04 The synthesis and application of hydroxamic acid in China began in the late 1960s and was used in industrial production in the 1970s. It has been used widely in the rare earth, cassiterite, copper oxide ore, iron ore, iron ore and titanium-tungsten ore flotation, a collector oxide ore promising. Therefore, research and analysis of its collection mechanism is of great significance for the promotion and application of this agent.
1 Tanzania niobium ore process mineralogy
The chemical formula of the coltan-niobite is AB 2 O 6 , which is referred to as coltan. A is iron and manganese , and B is strontium and barium. Different forms from pure bismuth to bismuth have a series of isomorphous structures characterized by an indeterminate ratio of iron to manganese. It contains Nb 2 O 5 from 1.97% to 78.88%, Ta 2 O 5 from 5.56% to 83.57%, MnO from 1.26% to 16.25%, and FeO from 1.89% to 16.25%. There are also similar materials such as Ti, Zr, W, TR, and U. In the group, the majority of the sputum is called coltan, and if it is the majority, it is called coltan. The crystal lattice of the mineral is a rhombic structure, and the space group is marked as Pcan. The structure consists of layers of A and B octahedrons. The same octahedron is connected as a chain in the layer by edges, and then connected to a common vertex. An A octahedral layer is connected to the adjacent B-octahedral layer by two vertices to form a BAB structure.
The bismuth iron ore iron ore is black, brownish black and reddish brown. The Mohs hardness is: coltanite 4.3 to 6.5; coltan is 6.5 to 7.2. The microhardness value of the coltan is 2400-8000 MPa, and the coltan is 8000-10700 MPa. The magnetic susceptibility of the bismuth iron ore-iron ore is (22.1-37.2) x 10 -6 . The tantalum iron has a dielectric constant of 10 to 12 and the tantalite has a density of 7 to 8. The density of minerals is 5.15 to 8.20 (increased as the content of strontium increases).
The lattice parameters of many minerals of the strontium iron ore-iron ore are related to the composition of the sample, and the fluctuation range is as follows: a = 0.5133 - 0.5054 nm; b = 1.445 - 1.405 nm; c = 0.5762 - 0.5683 nm. The atomic spacing in the manganese ore is Mn-O = 21.2 to 21.4 nm, and Ta-O = 18.6 to 21.2 nm. The atomic spacing of Mn-O is larger than that of Ta-O, so in the flotation solution, the manganese layer on the surface of the fracture surface of the yttrium manganese ore is relatively exposed to the action of benzoxamic acid ions. Therefore, Mn 2+ is considered to be the main activity center. [next]
2 The nature of the benzoate fatty acid
Benzohydroxamic acid, also known as benzoxamic acid, refers to two tautomers, namely:

These two tautomers are inseparable and their action can be attributed to either of the two isomeric forms.
In the presence of a mineral acid, hydroxamic acid is readily hydrolyzed to hydroxylamine and carboxylic acid; in a strong acid medium, Losen rearrangement occurs:
 [next]
[next]
Hydroxamic acid or its alkali metal salt can form a chelate with metal ions such as Cu 2+ , Fe 3+ , Fe 2+ , Zn 2+ , Mn 2+ . The structures of these chelates are as follows: (Me-metal ion )

The structure of the chelate varies with the type and action conditions of the metal ions. Most of them may be O- and 5-membered rings, and may also form O- and N-membered rings. So far, various metal oxygens have been given in the literature. The chelating structure of citrate is based on its physicochemical properties and is not a positive structural proof derived from reliable research methods.
At the same time, these metal chelates are very stable, which is the main reason for the recovery of minerals by hydroxamic acid.
3 Mechanism of action of benzoic acid and strontium manganese
3.1 Chelation and products
Based on the molecular orbital theory, the net charge (Q r ) and electron density (q r ) of each atom in the benzene hydroxamic acid and its tautomeric benzoxamic acid functional group are calculated, and the bonding positions are discussed.

(a) in the structural formula, the conjugate II 6 4 is composed of C and O, each π electron, and each of N and O;
(b) The conjugated body II 6 4 in the structural formula is composed of C and N each of π electrons, and each of O and O is an electron pair. [next]
Table 1 Net charge and electron density of each atom
Tab 1 The net electric charge of atom and electronic density
Electron density q r | q 1 =0.8439 q 2 =1.7876 q 3 =1.4438 q 4 =1.9244 | q 5 =0.9105 q 6 =1.8026 q 7 =l.3969 q 8 =1.8904 |
Net charge Q r | Q 1 =0.1561 Q 2 =0.7876 Q 3 =0.5562 Q 4 =0.9244 | Q 5 =0.0895 Q 6 =0.8026 Q 7 =0.3969 Q 8 =0.1096 |
It can be seen from the calculation results that in (a) structural formula, the distribution of electron density and electron density in the polar group of benzoxamic acid, the negative charge is concentrated on the O(2), O(4) atom, which will pass The O and O atoms in the polar group are bonded to Mn 2+ , that is, they are bonded to the Mn 2+ of the surface of the yttrium manganese ore by the O and O atoms in the polar group to achieve chemisorption. purpose.
In the structural formula (b), the negative charge of the thioxamic acid polar group is concentrated on the O(6), N(7) atom, which is bonded to the Mn 2+ through the N and O atoms in the polar group. That is to say, it will achieve the purpose of chemisorption by the N, O atom in the polar group bonding with the Mn 2+ of the surface of the yttrium manganese ore.
From the spatial factor analysis of quantum chemistry, since the spatial distribution of hydroxamic acid is different from that of hydroxamic acid, it is a non-planar distribution, and its two oxygen atoms are on the same side in space, and space factors form O and O chelation. favorable. The overlap of OH in hydroxamic acid is less than that of NH in hydroxamic acid, which is more favorable for OH to dissociate hydrogen, which facilitates chelation of oxygen with metal ions. However, N and O in hydroxamic acid are chelated with metal ions, and a tetraatomic cyclochelate is formed from the geometric structure. Since the tension of the four-membered ring is large, the stability of forming the N and O chelate is poor. The tendency of N and O chelation is small, while the five-membered ring chelate formed by O and O chelation is relatively stable.
Since the two isomers are mainly hydroxamic acid, its concentration in the solution is large, and the two metal chelate formed on the surface of the antimony ore have good stability of the five-membered ring chelate. . Therefore, the surface of the strontium manganese ore should be dominated by the action of benzoxamic acid and metal ions to form a five-membered ring chelate. [next]
3.2 Infrared spectroscopy results of benzoic acid monomanganese ore
Single mineral and actual ore flotation experiments confirmed that carbamic acid is a good collector for strontium manganese. The optimum pH range for the collection of bismuth manganese ore by benzoate is 6-10. The adsorption amount of benzyl hydroxamic acid on the surface of strontium manganese ore is closely related to the pH value, and the adsorption amount is the largest in the range of pH 7-10, which is consistent with the optimal flotation pH range. In order to further find out the product of benzoic acid on the surface of strontium manganese ore and its adsorption form, infrared spectroscopy was used to further study the effect of benzoquinone on the absorption of strontium manganese.
Figure 1 (a) is an infrared spectrum of benzoxamic acid. In the figure, the result of superposition of 3293.02 cm -1 and OH stretching vibration peaks is a characteristic peak of hydroxamic acid, 3062.15 cm -1 is an NH-based stretching vibration peak, and 2756.39 cm -1 is a -H stretching vibration peak. Due to the conjugation effect, a characteristic peak of the benzene ring skeleton appeared at 1556.44 cm -1 , 1492.20 cm -1 and 1453.38 cm -1 . 1076.42cm -1 , 1041.22cm -1 and 1022.09cm -1 are the three absorption peaks of NO vibration splitting, 1160.38cm -1 is CN stretching vibration peak, 1648.13cm -1 is -HC =N- stretching vibration peak, 693.09cm -1 , 535.39 cm -1 , and 427.60 cm -1 may be generated by the out-of-plane bending vibration of the benzene ring CH.
Figure 1 (b) is an infrared spectrum of benzoic acid via manganese citrate. The manganese benzoate is a product obtained by reacting benzoic acid with manganese sulfate at a pH of 5 to 6, and the product is obtained by repeatedly rinsing and drying the deionized water. Comparing Fig. 1(a), it is found that the NH and OH stretching superimposed vibration peaks have moved to 3418.26 cm -1 , and the NH - based stretching vibration peak has been shifted to 2281.32 cm - 1 - HC = N- stretching vibration peaks are obviously shifted to low wavenumbers, and have been shifted. At 1556.21 cm -1 , the CN stretching vibration peak was shifted from 1160.38 cm -1 to 1153.61 cm -1 , and the characteristic peak of the benzene ring skeleton also shifted to the low wave number. The test results show that benzoxamic acid has been combined with Mn 2+ to form a cyclic chelate.
Figure 1 (c) is an infrared spectrum of yttrium manganese ore. 1093.47cm -1 , 1030.36cm -1 , 865.78cm -1 , and 477.12cm -1 are characteristic peaks of strontium manganese ore.
Fig. 1(d) is an infrared spectrum of the yttrium manganese ore after the action of benzoquinone at pH 5-6. Comparative FIG 1 (c) found a new absorption peak appears 3299.34cm -1 in the range of 2000 ~ 4000cm -1, and it is NH 0-H stretching vibration peak is superimposed, within the scope of 1000-2000cm -1 there are two distinct The absorption peaks, 1644.47 cm -1 and 1564.22 cm -1 are -HC=N- stretching vibration peaks and benzene ring skeleton characteristic peaks, respectively. [next]
According to the results of infrared spectroscopy, it is known that the absorption peak of the yttrium manganese ore after the action of benzyl hydroxamic acid is basically the same as that of the manganese benzoate, so it can be considered that the benzoic acid will first crystallize with the yttrium manganese ore. The Mn 2+ integration of the lattice forms a manganese benzoate adsorption layer on the surface of the strontium manganese ore. The chelating structure is:


Fig.1 Infrared spectrum of benzoic acid in Niken manganese mine [next]
Fig 1 IR spectrophotometer of benzylhydroxmic acid and columbite-tantalite
4 Conclusion
Benzohydroxamic acid has a strong ability to capture manganese ore. The benzoxamic acid chelate with the manganese ion on the surface of the strontium manganese ore, and mainly forms a five-membered ring chelate, which is a surface chemical reaction and chemical adsorption. According to the crystal structure characteristics of strontium manganese ore, it is considered that Mn 2+ is the main flotation active center of strontium manganese ore.
references
[1] Zhang Zhixiong Oreology [M]. Beijing: Metallurgical Industry Press, 1981, 102-103
[2] Wang Wei, Pan Zhaoyu, Weng Lingbao et al. Systematic mineralogy (Volume 1) [M]. Beijing: Geological Publishing House, 1982, 560-561.
[3] Wang Dianzuo. Principle and application of flotation reagents [M], Beijing: Metallurgical Industry Press, 1982, 154-155.
[4] Li Shida, Tao Yuanqi, Huang Qianguang et al. Preliminary study on the de novo method of flotation reagent hydroxamic acid [J]. Nonferrous Metals ( Selection Part), 1993, (2): 16-19.
[5] Peng Shiwen, Liu Gaokui. Atlas of mineral infrared spectrum [M] Beijing: Science Press, 1982.122-123.
IN VESTIGATION OF ACTION MECHANISM BETWEEN BENZYLHYDROXIMIC ACID AND COLUMBITE-TANTALITE
QIU-Xianyang, GAO Yude
(Guangzhou Research Institute of Nonferrous Metals, Guangzhou 510651, China)
ABSTRACT
The test results indicating: Mn 2+ of the columbite-tantalite surface chelated with benzylhydroxmic acid to form five ring chelate matter. chemistry adsorption is the most important action.According to The crystal structure characteristic of columbite-tantalite, Mn" is thought as primary flotation active center.
KEY WORDS: columbite-tantalite; benzylhydroxmic acid; chelate action;
Auto Meat Strips Processing Line is latest design of Darin, which could produce pet snacks, suitable for pet food without casing directly baked meat and meat products, widely used in pet food article beef, article chicken, beef rod, chicken rod production and processing.
1, It is first processing line to produce meat snacks automatically and continuously.
2, The diameter and shape of meat strips can be various by changing the molds.
3, Fully controlled by PLC and touch screen to reduce the labor cost, 2-3 workers are needed to operate the whole line.
4, The characteristics is discharging speed, output evenly, can infuse bowel flesh lump, can also be used to fill the mi bowel, flexible, convenient and free loading, high efficiency, continuous production capacity, and cleaning convenient, suitable for various types of production enterprises.
5, The equipment is made from high quality 304 stainless steel, corrosion resistance, with high quality in intensity, reliable and durable, convenient cleaning, adopt gear two-way extrusion type structure, with high pressure, rotation smoothly.
6, Equipped with Auto Tray Loading System to feed the tray to load the meat strip automatically.
7, Safely cutting system to ensure the worker's safety, also same size of meat strips.
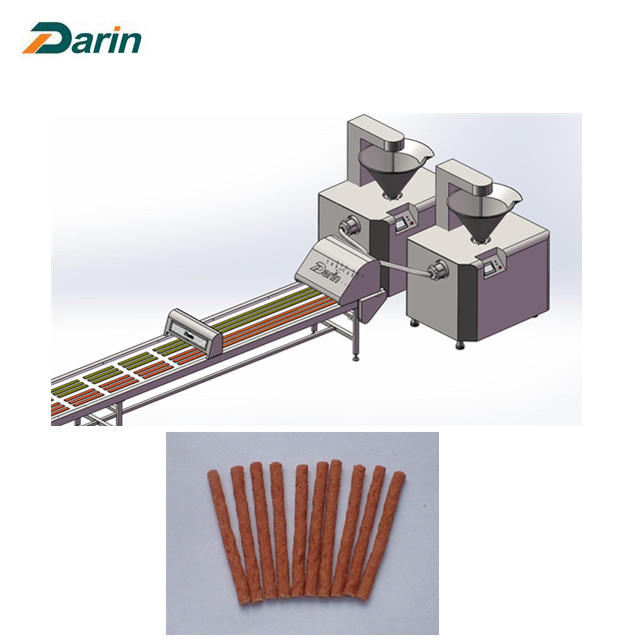

Auto Meat Strip Processing Line
Jerky Treats Stick Machine,Auto Meat Strip Processing Line,Meat Stick Making Machine,Dog Chewing Stick Machine
Jinan Darin Machinery Co., Ltd. , https://www.globaldarin.com

 [next]
[next] 




